Sulfur dioxide gas (SO2) is prepared in the laboratory in
following ways :
Theory : Sulfur dioxide gas is prepared in the
laboratory by heating copper turnings with conc Sulfuric acid (H2SO4) .
Reaction involved :
Cu + conc H2SO4 ----------- > CuSO4 + SO2 + H2O
H2SO4 ----------
>SO2 + H2O + [ O ]
Cu + [ O ] ---------- > CuO
CuO + H2SO4 ---------- > CuSO4 + H2O
Cu + [ O ] ---------- > CuO
CuO + H2SO4 ---------- > CuSO4 + H2O
Cu + conc 2 H2SO4 ----------- > CuSO4 + 2 H2O +
|
Procedure :
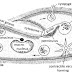
As shown in the figure, some copper turnings are kept in the round
bottom flask which is fitted with thistle funnel and delivery tube.
The other end of delivery tube is kept in a absorption bottle containing conc sulfuric
acid. Conc Sulfuric acid is poured in the thistle funnel which reaches to the
round bottom flask to start the reaction. Again , one end of delivery tube is
kept in absorption bottle and other end is introduced to an erect gas jar for
the collection of sulfur dioxide gas by upward displacement of air.
The copper or
flask is heated. On doing so the reaction between acid and cupper takes places
and sulfur dioxide gas (SO2) is produce in the flask. The SO2 gas produced is
not pure so it passed through absorbtion bottle containing con H2SO4 to
remove moisture from the gas as H2SO4 is a great dehydrating agent. After
removement of moisture finally, SO2 gas is collected in the gas jar by upward
displacement of air.
Test :
The presence SO2 gas
can be tested by introducing potassium manganese to the mouth of the jar. If it
discolourizes then we can know that SO2 is collected in the jar.






Thanks a lot...
ReplyDeleteThanks a lot...
ReplyDeleteYou are welcome samrat :)
ReplyDelete