HSEB Chemistry XI (Laboratory methods and Kipp's apparatus)
Hydrogen Sulfide is needed frequently in laboratory but intermittently for precipitation of basic radical which is done with the help of Kipp's apparatus
 |
| kipps apparatus |
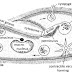
As shown in figure, upper bulb A has tappering end which reach to the bottom of bulb C. Bulb B is interconnected with bulb C. It has an outlet with a tap which can be opened and closed. Some iron sulfide is kept in the bulb B. Dil acid is passed in the funnel which reach to the bottom of bulb C and again raises up to meet with iron sulfide. When the acid and FeS, H 2 S gas is produced which can be collected by open which opeing the tap.
When H 2 S gas is not needed , the tap is closed. The gas continues in bulb b as tap is closed. The pressure of gas increases which pushes the acid from bulb b to bulb c which again returns back to the bulb A. Now the acid is not in contact with FeS so H 2 S gas will not be produced.
When tap is opened, reverse phenomenon occurs.





0 comments: