More on original site : Manufacture of Ammonia by Haber's process
Principle : In this process , Ammonia is prepared from nitrogen and hydrogen. In ordinary condition , nitrogen do not react with hydrogen but when it is mixed with hydrogen in the ration 1:3 under 450 C temperature and 200-500 atm pressure in presence of Iron as catalyst and Molybdenum as promoter then it combines to form
Principle : In this process , Ammonia is prepared from nitrogen and hydrogen. In ordinary condition , nitrogen do not react with hydrogen but when it is mixed with hydrogen in the ration 1:3 under 450 C temperature and 200-500 atm pressure in presence of Iron as catalyst and Molybdenum as promoter then it combines to form
Ammonia (NH3).
The reaction is exothermic,
reversible and proceeding with decrease in volume. So, following condition is
needed for better yield of it :
a. Low temperature : The reaction is exothermic , it as a rule
is followed by low temperature. But if the temperature is very low then the
rate of reaction will
be very slow and
production of Ammonia will not be economically fruitful and beneficial. So
optimum temperature of 450 C is maintained in this process.
b. high pressure : The reaction is reversible so it favored by high pressure. So pressure
of about 200-500 atm is maintained.
c. High concentration : If the reactant are excess the the product formed will also be
same . So either hydrogen or nitrogen in the reactant side is made excess
to
produce high amount
of ammonia.
d. Use of catalyst : In absence of catalyst, the reaction will be slow. So, Iron is
used as catalyst along with molybdenum as promoter to increase the rate of
reaction
without hampering
it.
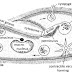
Process : In this process, N2 is treated with H2 in
the ratio 1:3 . Then the gaseous mixture is passed through compressor in which
pressure of 200-500 atm is applied.
Then the gases
mixture is passed through catalyst chamber packed with finely divided Iron + molybdenum.
The chamber is initially heated to about 450 C to initiate the
reaction. During
this condition , 15% of the gaseous mixture is turned into ammonia. Thus , the
gaseous coming out of the chamber contains NH3 and unreacted H2 and N2. These
are then passed through condenser to condense only ammonia not H2 and N2. The liquefied
ammonia is collected at the bottom from time to time. The other
uncombined
H2 and N2 is recycled and reprocedded.






Shakti Chemicals is a leading Manufacturer and exporters of ammonium bisulfite Solution. Shakti Chemicals is an ISO 9001-2015 company following all standard strictly to conserve our quality of products. Contact Us: 9825043369
ReplyDelete