Hydrogen sulphide gas can be prepared in the laboratory in following ways :
Theory : Hydrogen sulphide gas can be prepared in the laboratory by reacting iron sulphide (FeS) with dilute non-oxidizing acid like HCl and H 2 SO 4 .
Reaction : FeS + dil H 2 SO 4 ---------- > H 2 S + FeSO 4
FeS + HCl ----------- > H2S + FeCl2
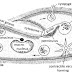
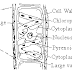
Some iron sulphide in kept in the Wolfe's bottle which is fitted with delivery tube and thistle funnel. The other end of delivery tube is introduced to an erect gas jar for the collection of H 2 S gas by upward displacement of air.
Dil acid is introduced to Wolfe's bottle through thistle funnel. Now the acid comes in contact with the FeS and H 2 S gas is produced which is collected in the gas jar.
Purification : The H 2 S gas produced in the laboratory may be contaminated with hydrogen gas or some acid spray. Thus, H 2 S is purified by passing the mixture of gas through aqueous suspension of Magnesia (MgO) . MgO absorbs H 2 S to for magnesium hydrosulphite , Mg(H 2 S) leaving behind hydrogen gas. When this Mg(H2S) is heated at the temperature of about 60-70 C, purified H 2 S gas is produced.
MgO + H 2 S ---------- > Mg(H 2 S) 2 + H 2 O
Mg(H 2 S) 2 ----------- > H 2 S + MgS
Theory : Hydrogen sulphide gas can be prepared in the laboratory by reacting iron sulphide (FeS) with dilute non-oxidizing acid like HCl and H 2 SO 4 .
Reaction : FeS + dil H 2 SO 4 ---------- > H 2 S + FeSO 4
FeS + HCl ----------- > H2S + FeCl2
Some iron sulphide in kept in the Wolfe's bottle which is fitted with delivery tube and thistle funnel. The other end of delivery tube is introduced to an erect gas jar for the collection of H 2 S gas by upward displacement of air.
Purification : The H 2 S gas produced in the laboratory may be contaminated with hydrogen gas or some acid spray. Thus, H 2 S is purified by passing the mixture of gas through aqueous suspension of Magnesia (MgO) . MgO absorbs H 2 S to for magnesium hydrosulphite , Mg(H 2 S) leaving behind hydrogen gas. When this Mg(H2S) is heated at the temperature of about 60-70 C, purified H 2 S gas is produced.
MgO + H 2 S ---------- > Mg(H 2 S) 2 + H 2 O
Mg(H 2 S) 2 ----------- > H 2 S + MgS






0 comments: